C&S Newsletters
C&S Newsletter No.51
- Date2024/09/30 16:55
- Hit 253

- Meaning of “new substance (substance with new chemical structure of active part showing medical effect)” required for inventions subject to patent term extension, Supreme Court decision 2021Hu11070
- Strengthening sanctions for technology theft under revised law
- Intelligent Internet of Things (AIoT) Technology for Prevention of Natural Disasters, led by Korea
- In 2023, the number of U.S. patent litigations filed by domestic companies increased by 3.9% year-on-year to 107 cases
- Last year, domestic intellectual property rights applications and registrations turned to an increase
- C&S News
Meaning of “new substance (substance with new chemical structure of active part showing medical effect)” required for inventions subject to patent term extension, Supreme Court decision 2021Hu11070
Woo-Taek Hwang, Patent Attorney
Soo-Kyung Chung, Patent Attorney
1. Overview
In order to practice an invention related to a medicine or the like, a license should be obtained under the Pharmaceutical Affairs Act. A patentee may suffer the disadvantage of not being able to practice the patented invention during the process of obtaining such a license. In order to remedy such disadvantage and protect and encourage the pharmaceutical inventions, Article 89 (1) of the Korean Patent Act provides a system that extends the patent term of the inventions that require a long period of time to obtain a license under the Pharmaceutical Affairs Act by the period during which the patented invention cannot be practiced. However, Article 89(1) of the Korean Patent Act and Article 7(1)* (hereinafter referred to as the ‘Enforcement Decree of this case’) of the old Enforcement Decree of the Patent Act (hereinafter referred to as the ‘Enforcement Decree of this case’) does not stipulate the definition of the ‘active part showing the medicinal effect’ or the specific scope of the inclusion thereof.
This article will introduce the Supreme Court decision that comprehensively considers the overall system and purpose of relevant laws, the regulation format and content of the relevant provisions based on the principle of faithfully interpreting the ‘active part showing the medicinal effect,’ the definition or the specific scope of which is not clearly stipulated in the law, according to the usual meaning thereof.
2. Issues
Based on the form and content of the provisions of the Enforcement Decree of this case, as well as the relevant pharmaceutical laws, the ‘active part showing the medical effect’ in the Enforcement Decree of this case denotes ‘the part of the active ingredient of a drug that is active and shows the efficacy and effect as stipulated in the product license of the drug by its inherent pharmacological action.’ On the other hand, there are cases in which a part that is not active in and of itself is combined with the ‘active part showing the medical effect’ of a previously licensed drug and affects the degree of efficacy and effect of the drug.
Peginterferon beta-1a, the active ingredient in the claim of this case (hereinafter referred to as the ‘drug of this case’), is a pharmaceutical invention in which polyethylene glycol (PEG) is covalently bonded to interferon beta-1a and PEGylated. The plaintiff (patentee) filed an application for patent term extension in this case, but the Examiner of the Korean Intellectual Property Office and the Patent Trial and Appeal Board (PTAB) rejected the application and dismissed the plaintiff's request for a trial to cancel the rejection on the grounds that there is an existing licensed drug containing interferon beta-1a as the active ingredient, and the drug in this case has the same active ingredient that shows the same therapeutic effect as the existing licensed drug, interferon beta-1a, and therefore, is not a new substance.
The Patent Court overturned the PTAB’s decision and stated that “the active part that shows the medical effect of the drug in this case is peginterferon beta-1a, and even if the existing licensed drug containing interferon beta-1a as the active part is considered, the polyethylene glycol bound to the interferon beta-1a is also a new substance with the chemical structure of the active part that shows the medical effect”.
That is, the PTAB and the Patent Court have different judgments as to whether the entire combination of a “non-active part” and an “active part showing the medical effect” of an existing licensed drug corresponds to the “active part showing the medical effect” as stated in the Enforcement Decree of this case.
3. Supreme Court 2021Hu11070 Decision
A. The Supreme Court interpreted the new substance (a substance with a new chemical structure of the active part that shows the medical effect) stipulated in the Enforcement Decree of this case, and held that since the form and content of the provisions of the Enforcement Decree of this case distinguish between the ‘active ingredient’ and the ‘active part that shows the medical effect,’ even if a part that is not active in itself is combined with the ‘active part that shows the medical effect’ to affect the degree of efficacy and effect of the pharmaceutical product, the entire combination cannot be considered as the ‘active part that shows the medical effect’ as stated in the Enforcement Decree of this case, and in light of the intent and purpose of the patent term extension system, it is difficult to consider as an invention subject to the patent term extension a pharmaceutical invention that adds a part that is not active in itself to the known active part of a previously licensed pharmaceutical product to affect the degree of efficacy and effect of the pharmaceutical product.
B. The Supreme Court concluded that an active ingredient of the drug in this case, which is active in the body and shows the therapeutic effect of relapsing multiple sclerosis through its inherent pharmacological action, is interferon beta-1a**, and a polyethylene glycol part bound to interferon beta-1a is only a part that affects the level of activity of interferon beta-1a by allowing the interferon beta-1a to remain in the blood for a longer period of time or by lowering the binding of interferon beta-1a to the protein receptor, while not showing the activity in the body or the therapeutic effect as mentioned above, and therefore, even if the ‘active part that shows the medical effect’ of the drug in this case is interferon beta-1a, and the polyethylene glycol is bound to the ‘active part that shows the medical effect’, interferon beta-1a, to form peginterferon beta-1a, the entire compound, peginterferon beta-1a, cannot be considered as the ‘active part showing the medical effect ’ as stated in the Enforcement Decree of this case, and the interferon beta-1a, which is the ‘active part showing the medical effect’ in the drug of this case, has the same three-dimensional chemical structure as the interferon beta-1a, which is the ‘active part showing the medical effect’ in the previously licensed drug; therefore, the Supreme Court reversed and remanded the case to the lower court, which made a judgment different therefrom.
4. Implications
The Supreme Court decision clarified the meaning of the ‘active part showing the medical effect’ in relation to the patent term extension of the pharmaceutical patents under the Korean Patent Act. Specifically, by clarifying that in cases in which a non-active part is combined, the entire combination cannot be considered to be the ‘active part showing the medical effect,’ a standard for the patent term extension of the pharmaceutical patents has been proposed.
This is expected to have a significant impact on pharmaceutical patent policy and the pharmaceutical industry, so that patent attorneys performing patent preparation and prosecution work should be familiar with the Supreme Court decision, and should simultaneously provide accurate examination information to their clients so that the pharmaceutical companies can carefully establish patent management strategies from the patent application stage, and should be guided to prepare for various scenarios to minimize legal risks.
* In order to practice a patent invention, the invention of a pharmaceutical product [limited to a pharmaceutical product manufactured with a new substance (refers to a substance whose chemical structure of the active part that shows the medical effect is new. The same applies hereinafter in this Article) as an effective ingredient and that has been licensed for use for the first time] that has been licensed for use in accordance with Article 31(2) and (3) or Article 42(1) of the Pharmaceutical Affairs Act is stipulated.
**Interferonbeta-1a, an active ingredient in pre-licensed drugs, is a protein drug that is active in the body and has the effect of treating recurrent multiple sclerosis by controlling abnormal immune effects.
Strengthening sanctions for technology theft under revised law
Jung-Won LEE, Attorney / Patent Attorney
The revised Patent Act and the Unfair Competition Prevention Act, which came into effect on August 21, 2024, mainly aim to increase the level of sanctions against infringers who steal the technology of patent holders and trade secret holders. A particularly notable change is the increase of the amount of damages for willful infringement up to five times. In this article, the main contents of the revised law and its significance will be discussed.
1. Increased Punitive Damages
The revised Article 128 of the Patent Act and Article 14(2) of the Unfair Competition Prevention Act allow the court to determine the amount of damages not exceeding five times the amount recognized as damages in the event of willful infringement of patent rights, trade secrets, or ideas. This revision increases the existing punitive damages from three times to five times, and accordingly, it seems that a higher level of sanctions will be possible for the acts of intentional technology theft.
Even in the United States, which is the most active in technology protection, punitive damages of up to two times in the trade secret infringement and up to three times in the patent infringement are allowed, and the only countries that allow punitive damages of five times are China and Korea. In Korea, the perception that it is more profitable to copy technology than to develop novel technology and secure patents or trade secrets with regard thereto has become widespread, and there are many cases in which the victimized companies give up the lawsuit because the amount of damages is insufficient even if they can win the lawsuit. However, it is expected that the revised laws may change companies' perceptions of technology protection.
2. Introduction of a corrective order system for unfair competition acts
Article 8(1) and Article 20(1) (1-2) of the revised Unfair Competition Prevention Act allow the Commissioner of the Korean Intellectual Property Office to directly issue corrective orders for unfair competition acts. Previously, the Commissioner of the Korean Intellectual Property Office could conduct administrative investigations and make corrective recommendations, but this has been criticized as ineffective due to lack of enforcement power. Accordingly, the revised law introduces a corrective order system, allowing the Commissioner of the Korean Intellectual Property Office to order the cessation of violations, removal or modification of signs, prevention of future recurrence, and includes provisions requiring those who fail to comply with corrective orders to publish the details of violations, or impose a fine of up to 20 million won.
In addition, Article 7-2 of the same Act allows the victims of technology theft to request inspection and copying of materials related to the Korean Intellectual Property Office’s administrative investigation of unfair competition in order to reduce the burden of securing evidence for the victims of technology theft, and according to Article 14-7 of the same Act, the court may restrict the scope of inspection of records and those who can view the records in order to protect trade secrets included in the administrative investigation records.
The current system is expected to improve the ability of the Commissioner of the Korean Intellectual Property Office to act against technology theft acts, and result in effective responses at the government level.
3. Strengthening the penalties for corporations
Articles 19 and 19-2 of the revised Unfair Competition Prevention Act increase the statutory sentence for corporations, allowing fines to be imposed up to three times the upper limit of fines that may be imposed on individuals, and extend the statute of limitations for unfair competition by corporations from 5 years to 10 years. Meanwhile, Article 18-5 of the Unfair Competition Prevention Act provides a means to stop infringing acts outside of civil procedures by allowing for the confiscation of objects that contributed to or resulted from infringing acts even in criminal proceedings.
Conventionally, the statutory penalties imposed on corporations was the same as that imposed on individuals, so that the level of penalties for large-scale technology theft at the corporate level was weak. In addition, victims could only stop infringement through civil proceedings known as infringement prohibition lawsuits. However, the revised law is expected to effectively block technology theft at the corporate level and enable stricter penalties.
4. Future Outlook and Tasks
This revision of the laws has brought about many institutional changes for the protection of patents and trade secrets. These changes are evaluated as groundbreaking measures that will further strengthen the protection of intellectual property rights. Companies should pay attention to these changes and pay more attention to acts of unauthorized use or theft of third-party technologies and reexamine their intellectual property strategies.
Intelligent Internet of Things (AIoT) Technology for Prevention of Natural Disasters, led by Korea
As natural disasters are rapidly increasing due to climate change, technologies that prevent disasters by converging IoT (Internet of Things) and AI (Artificial Intelligence) technologies are gaining attention.
According to the Korean Intellectual Property Office (KIPO)’s analysis of global AIoT-based disaster prevention patents filed in IP5, the number of applications for technologies that use the Intelligent Internet of Things (AIoT), which combines IoT and AI technologies, to prevent disasters, such as floods, has increased by an average of 19.5% per year over the past 10 years (2012 to 2021).
AIoT-based natural disaster prevention technologies collect big data, such as satellite data, weather data, and IoT sensor data, predict damage situations through AI learning, and provide location-based evacuation routes.
By disaster type, the geological disaster field had the largest number of applications at 51.4%, followed by flood damage field (23.9%), meteorological disaster field (17.0%), and marine disaster field (7.7%)
The KIPO stated that natural disasters due to abnormal climate events are increasing, but the development of natural disaster prevention technology through AIoT is expected to contribute to reducing damage caused by natural disasters.
Source: Press release from KIPO (June 21, 2024)
In 2023, the number of U.S. patent litigations filed by domestic companies increased by 3.9% year-on-year to 107 cases
Last year, 107 patent litigations were filed between domestic companies and foreign companies in the U.S., up 3.9% on a year-on-year basis.
The Korean Intellectual Property Office (KIPO) analyzed that since domestic small and medium-sized companies actively exercised their patent rights against foreign companies, the number of patent litigations related to small and medium-sized companies increased from 28 cases in 2022 to 34 cases in 2023, and among the number of patent litigations, the number of patent litigation filed by the domestic companies was higher than the number of patent litigations filed against the domestic companies.
On the other hand, patent litigations related to large companies decreased from 75 cases in 2022 to 73 cases in 2023.
By industrial sectors, U.S. patent litigations by domestic companies still mainly occurred in the electrical and electronic fields such as computers, telecommunications, and semiconductors in 2022 and 2023. Last year, patent litigations in the electrical and electronic fields accounted for 85 out of 107 cases, or 79.4%.
Among the 107 U.S. patent litigations involving domestic companies last year, only 23 patent litigations (21.5%) were filed by domestic companies, and 84 patent litigations (78.5%) were filed by the other parts, accounting for the majority of patent litigations.
Source: Press release from KIPO (June 28, 2024)
Last year, domestic intellectual property rights applications and registrations turned to an increase
Last year, the number of intellectual property rights applications and registrations in Korea increased again after a slight decrease in 2022.
Patent applications increased by 2.4% as compared to the previous year, and in particular, patent applications from universities and public research institutes increased by 9.1%, and large corporations and small and medium-sized enterprises increased by 7.6% and 3.9%, respectively. In contrast, trademark and design applications decreased by 1.5% and 2.3%, respectively, showing a continued downward trend in 2023 following 2022. This is interpreted as a result of a decrease in trademark applications in industries with fewer startups in 2023.
In terms of intellectual property rights registrations, patents and designs decreased as compared to the previous year, but trademarks increased significantly by 28.6%, increasing the overall domestic intellectual property rights registrations.
International patent applications (PCTs) have been decreasing in most countries last year except for Korea, France, and the Netherlands, but Korea ranked 4th in the world with a 1.2% increase as compared to the previous year.
Source: Patent News (August 27, 2024)
C&S News
New Patent Attorneys
C&S Patent and Law Office has recently hired new patent attorneys to further strengthen our business capabilities in the fields of machinery, electronics, and materials. We will continue to do our best to provide professional services by recruiting talented employees.
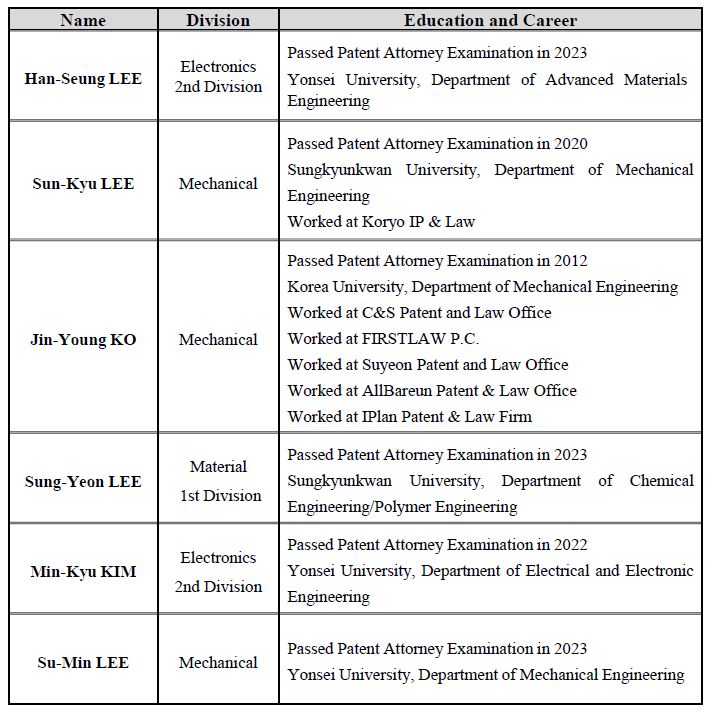
-
 FILE 1
51thNewsletterEN.pdf
(byte : 423.0K / DOWNLOAD : 196)
FILE 1
51thNewsletterEN.pdf
(byte : 423.0K / DOWNLOAD : 196)

